ORIGINAL ARTICLES
INTRODUCTION. Pharmacovigilance ensures drug safety through continuous monitoring, yet awareness remains limited in low-resource settings. Ranitidine, a widely used antacid, was withdrawn globally in 2020 due to contamination with N-nitrosodimethylamine (NDMA), a suspected carcinogen. Despite an official withdrawal from drug circulation, ranitidine is still at risk of being used unreasonably; the conditions given make it a relevant goal to assess professional awareness of pharmaceutical employees.
AIM. This study aimed to detect pharmacovigilance challenges among Lybian pharmacies and explore the new growth vectors using awareness of ranitidine withdrawal reasons and safety issues among pharmacy employees as an example.
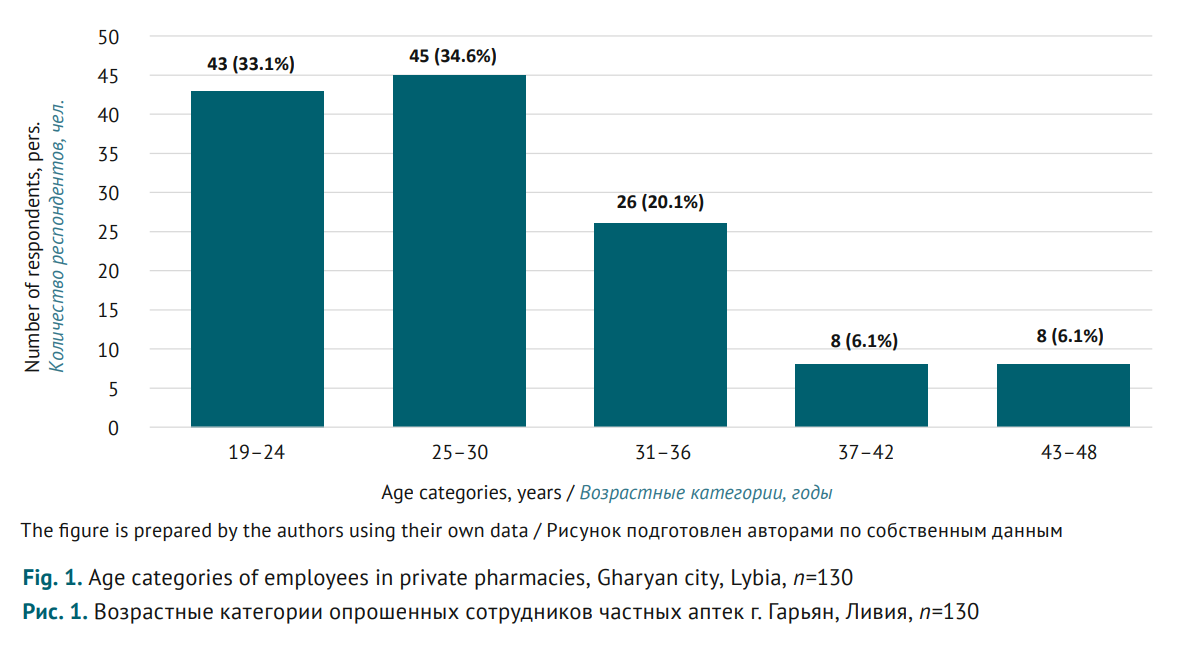
MATERIALS AND METHODS. A cross-sectional study was conducted engaging 130 pharmacy employees of Gharyan city, Libya, in January-March 2023. A structured questionnaire with three sections was used, including: 1) demo graphics (age, gender, education, and experience); 2) basic pharmacovigilance knowledge of participants (six questions with Yes/No answers); 3) awareness of ranitidine withdrawal (six questions with Yes/No answers). Data were analysed using descriptive statistics.
RESULTS. The survey showed that almost a third of participants (36.2%) were not familiar with the term “pharmacovigilance”; more than two-thirds (63.8%) did not know that Libya’s national pharmacovigilance centre existed. This contradicts the responses regarding pharmacovigilance measures: 78.5% stated they were trained in pharmacovigilance; 81.5% knew about special report forms to be filled out for any adverse drug reactions, while 16.2% previously made reports on adverse reactions. 55.4% of participants were familiar with the cases where drugs were withdrawn due to related risks. At the same time, 79.2% thought that ranitidine was still dispensed from the pharmacies; and 30.8% misclassified ranitidine, a Н2-blocker, as an antihistamine. 61.5% of participants thought carcinogenic impurities were caused by manufacturing contamination; moreover, only another 36.1% associated the impurities also with the improper storage.
CONCLUSIONS. The identified critical gaps in pharmacovigilance knowledge and ranitidine safety highlight the need for targeted educational interventions among Gharyan pharmacy employees and regulatory enforcement of drug withdrawals from the circulation.
REVIEWS
INTRODUCTION. Lamotrigine (LTG) is among the most commonly prescribed second-generation antiepileptic drugs due to its low teratogenic risk. However, lamotrigine has pronounced neurotoxic, hepatotoxic, dermatotoxic potential (for genetic and metabolic causes) and in some cases can even cause multi-organ failure. Understanding lamotrigine mechanism can help individualise therapy and increase its safety, considering pharmacodynamics and pharmacometabolomics that determine its metabolism, transport, and elimination in a particular patient.
AIM. This study aimed to develop an approach to lamotrigine therapy of epilepsy and other neurological and psychiatric diseases reducing neurotoxicity, with due regard to pharmacogenomics and pharmacometabolomics.
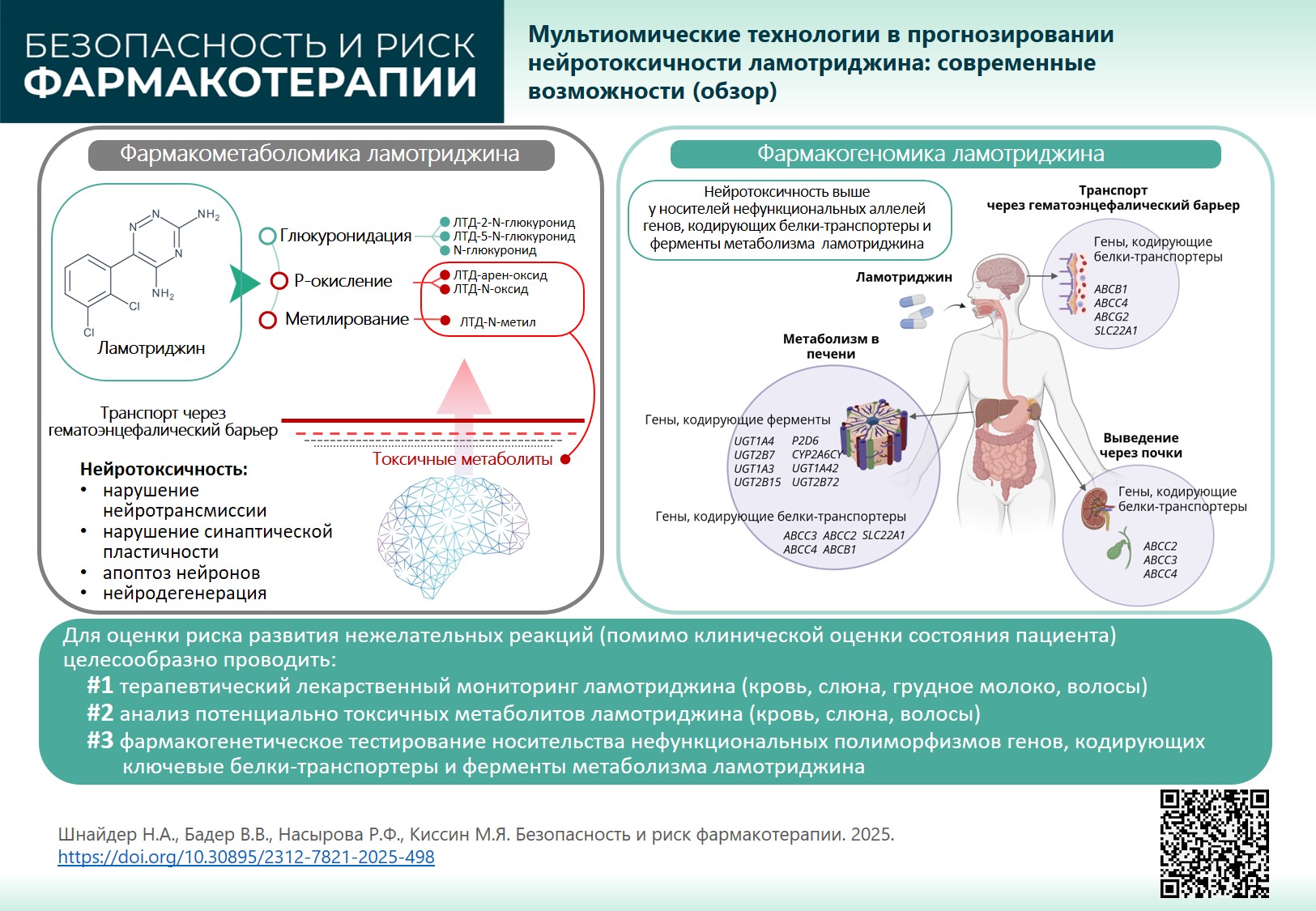
RESULTS. LTG is metabolised in the liver in two pathways: glucuronidation (major pathway) and P-oxidation (minor pathway). As a result, neutral and toxic (reactive) lamotrigine metabolites are produced that can circulate in blood serum for a long time, penetrate the damaged blood-brain barrier in patients with therapy-resistant seizures and have a neurotoxic effect, triggering or maintaining neurotransmission disorders, impaired synaptic plasticity, neuronal apoptosis and other neurodegeneration mechanisms. An important role in lamotrigine neurotoxicity belongs to transport proteins involved in the efflux (excretion) of reactive (toxic) metabolites from the brain into the systemic circulation, as well as from hepatocytes into the gastrointestinal tract by bile and through the kidneys with urine. Genetically determined delayed efflux through the blood-brain barrier (pharmacogenomics) increases lamotrigine neurotoxic potential.
CONCLUSION. To assess the risk of lamotrigine-induced adverse reactions, together with clinically assessing patient’s condition, it is recommended to: 1) monitor drug distribution (blood, hair, saliva, breast milk); 2) analyse potentially toxic metabolites (blood, saliva, hair); 3) perform pharmacogenetic tests for non-functional polymorphisms of genes encoding key transport proteins and enzymes involved in drug metabolism. Results of pharmacogenetic and pharmacometabolic tests applied in the clinical practice of an epileptologist will allow to manage lamotrigine neurotoxiсity.
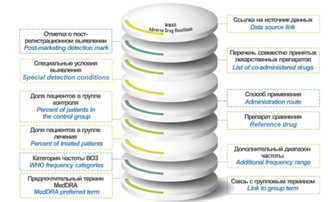
INTRODUCTION. Evaluating safety data on already registered drugs is an important stage in medicine development. Databases (DBs) of adverse drug reactions (ADRs) have been created and maintained by regulatory authorities and individual research groups. The data aggregated in such DBs can help create reference and training datasets for experimental and in silico studies. A preliminary assessment and systematisation of information from these databases can facilitate the selection of DBs relevant to a specific study.
AIM. This study aimed to assess applicability of public ADR databases for safety studies of medicinal products.
DISCUSSION. Eleven public DBs that provide information on ADRs of approved drugs were studied: FAERS, DAEN, MedEffect Canada, EudraVigilance, VigiBase, SIDER, MetaADEDB, ADReCS-Target, T-ARDIS, OnSIDES, and WWAD. The differences between these databases are primarily due to the variety of sources they use: spontaneous reports (FAERS, DAEN, MEDEFFECT, EudraVigilance, VigiBase), patient information leaflets and other official documents (SIDER, OnSIDES, WWAD), scientific publications (ADReCS-Target), and other open-access web resources (MetaADEDB, T-ARDIS). All the reviewed databases can be used for informational support and analysis of drug safety profiles. SIDER, MetaADEDB, ADReCS-Target, OnSIDES, and WWAD are useful in preclinical studies, particularly while developing training sets for in silico methods. Hypotheses for possible ADR mechanisms and search for new drug repurposing vectors can be arranged using ADReCS-Target, WWAD, and T-ARDIS, since these DBs provide additional data on active pharmaceutical substances or target molecules. Using computer-aided methods without thorough hands-on search in the DBs such as ADReCS-Target, T-ARDIS, OnSIDES, and MetaADEDB, limits their applicability in tasks requiring accurately analysed information.
CONCLUSIONS. The DBs reviewed can serve as a valuable tool for addressing a wide range of biomedical issues. To select a DB relevant for a specific study, it is important to consider the underlying principles, since varying sources and annotation methods can affect the reliability of results.
ISSN 2619-1164 (Online)