MAIN TOPIC: THE DARK SIDE OF PHARMACOTHERAPY: ADVERSE DRUG REACTIONS
INTRODUCTION. Oral mesalazine (5-aminosalicylic acid) products are commonly used to treat inflammatory bowel disease, in particular, ulcerative colitis. The clinical efficacy of these medicinal products depends directly on the composition and properties of the polymers used to deliver mesalazine to the affected areas of the colon. However, the information that has been accumulated to date suggests that the release of mesalazine from enteric-coated dosage forms in gastrointestinal tract simulations differs from that in the actual human gastrointestinal tract, which necessitates further research.
AIM. This study aimed to systematise information on the polymers used in the enteric coating of mesalazine products and to assess the pharmaceutical risks associated with the potential reduction in the efficacy of ulcerative colitis therapy.
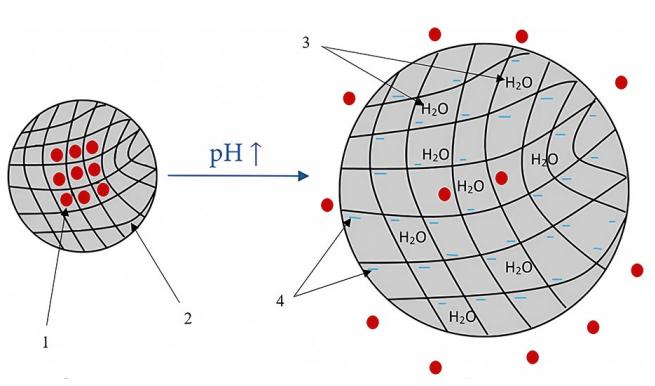
DISCUSSION. The absorption and metabolism of mesalazine dictate the need for enteric-coated dosage forms to deliver the active substance directly to the affected areas of the colon. The most common polymer used in the manufacturing of oral mesalazine products is a methacrylic acid–methyl methacrylate copolymer with a monomer ratio of 1:1, which releases the active substance at pH 7.0. Some manufacturers use a methacrylic acid–ethyl acrylate copolymer with a monomer ratio of 1:1, which dissolves at pH 5.5. The gastrointestinal pH in patients with inflammatory bowel disease may vary in wide and often overlapping ranges depending on the organ (1.0–7.0 in the stomach, 5.0–6.2 in the duodenum, 6.1–7.1 in the jejunum, 7.4–7.5 in the ileum, and 5.7–7.5 in the colon with a possibility of acidification in ulcerative colitis patients). The rate of gastrointestinal transit varies widely as well. These factors may cause premature release of mesalazine in the stomach or the small intestine before the dosage form reaches the colon, which poses the risks of reduced clinical efficacy and systemic adverse effects.
CONCLUSIONS. In the vast majority of ulcerative colitis patients, the methacrylic acid–methyl methacrylate copolymer provides targeted delivery of 5-aminosalicylic acid from tablets and granules, facilitating its local action in the colon. However, developers and manufacturers selecting the polymer for enteric coating of oral mesalazine dosage forms should consider the pharmaceutical risks associated with reduced clinical efficacy.
INTRODUCTION. The high risk of life-threatening ventricular arrhythmias, particularly Torsade de Pointes (TdP), makes QT prolongation one of the most significant adverse drug reactions (ADRs) due to cardiotoxicity associated with antipsychotics (APs).
AIM. This study aimed to systematise information about AP effects on the QT interval duration and TdP risk in patients with mental disorders and to provide recommendations on preventive measures for practising psychiatrists and clinical pharmacologists.
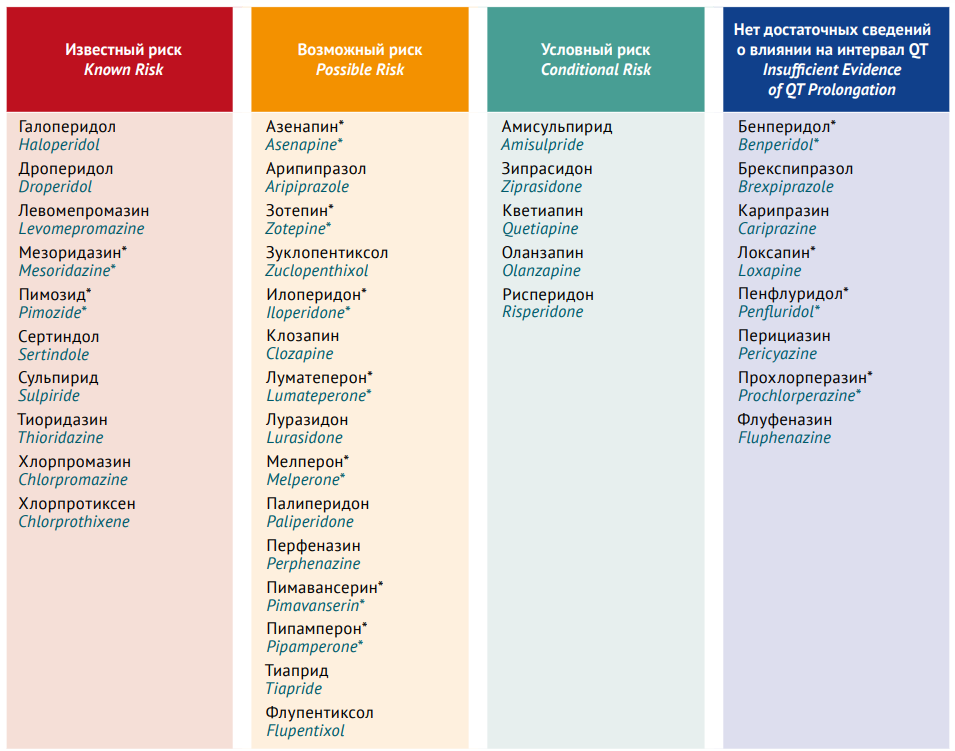
DISCUSSION. The authors searched information in PubMed, eLIBRARY.RU, and Google Scholar. The analysis included full-text articles on the results of placebo-controlled studies, crossover studies, case–control studies, systematic reviews, meta-analyses, and Cochrane reviews published from 1 September 2013 to 30 September 2023. The main mechanism of AP cardiotoxicity is the inhibition of voltage-gated ion channels (primarily potassium channels) in the cardiomyocyte membrane. Most first-generation APs are associated with dose-dependent QTc prolongation; thioridazine, chlorpromazine, and levomepromazine pose the highest risk of QTc prolongation and TdP. The results of this review do not support the hypothesis of a lower risk of QTc prolongation with next-generation APs than with first-generation APs. The correlation between serum AP levels and QTc prolongation severity is less characteristic of secondand third-generation APs. However, all second-generation APs lengthen the QTc interval and increase the risk of TdP, with clozapine and olanzapine posing the highest risk. Depending on the risk of QTc prolongation, APs can be divided into 3 groups: low-risk products (aripiprazole, lurasidone, cariprazine, paliperidone, and zuclopentixol), moderate-risk products (quetiapine, perphenazine, fluphenazine, olanzapine, clothiapine, and haloperidol), and high-risk products (chlorpromazine, promazine, clozapine, levomepromazine, and ziprasidone). The relationship between AP-induced QTс prolongation and TdP is ambiguous. If an AP exerts a homogeneous effect on cardiomyocytes, the risk of TdP remains low despite significant QTс prolongation.
CONCLUSIONS. The summarised data on AP effects on QT interval duration and TdP risk in patients with mental disorders as well as the proposed recommendations for reducing TdP risk may be in demand by psychiatrists and clinical pharmacologists selecting AP and may help minimise the likelihood of potentially fatal AP-induced arrhythmogenic cardiac ADRs.
INTRODUCTION. Low-dose methotrexate (less than 30 mg/week) is the standard therapy for rheumatic diseases. Methotrexate overdose due to errors by patients or medical staff may lead to severe complications and life-threatening conditions.
CASE DESCRIPTION. This article presents a retrospective analysis of the medical records of three clinical cases of methotrexate overdose with toxic reactions (one probable and two confirmed cases) observed in rheumatology patients at general and preventive medicine clinics in the Kaliningrad and Smolensk regions in 2019–2024. The analysis examined the clinical presentation of methotrexate overdose, medical history, concomitant therapy, laboratory findings, and patient management strategy from the time of admission. All patients had confirmed rheumatic diagnoses, including psoriatic arthritis (one male, 59 years old) and seropositive rheumatoid arthritis (two females, 68 and 57 years old). As a baseline anti-inflammatory therapy, patients received methotrexate at a dose of 10–20 mg/week with the mandatory addition of folic acid at a dose of at least 5 mg/week. Patients had concomitant renal, cardiovascular, and metabolic disorders. The primary cause of these overdose cases was that the doses prescribed for weekly administration were taken daily. Overdose manifested as haemorrhagic syndrome, erosive and ulcerative mucosal lesions, dyspepsia, and changes in laboratory findings. In two confirmed overdose cases, adverse drug reactions manifested 13 and 14 days after the start of erroneous methotrexate administration. Two patients died on days 4 and 7 from admission, and one patient recovered and was discharged after 40 days of hospital stay.
CONCLUSIONS. Methotrexate overdose can lead to haematopoietic, gastrointestinal, cutaneous, and mucosal disorders and result in life-threatening conditions and even death. The described cases emphasise the importance of clearly informing patients of methotrexate dosing regimens and possible symptoms of overdose. This approach can mitigate the potential risk and adverse consequences of overdose while improving the safety of outpatient methotrexate treatment for rheumatic diseases.
INTRODUCTION. The high mortality and disability rates associated with cold injury are often a consequence of urgent amputation or disarticulation surgery. The use of the Russian gene therapy product Neovasculgen can decrease the amputation rate in patients with chronic limb ischaemia through preventing the progression of endothelial dysfunction and stimulating tissue revascularisation. The mechanism of action of Neovasculgen indicates its potential as an initial treatment option for patients presenting with frostbite and progressive tissue ischaemia.
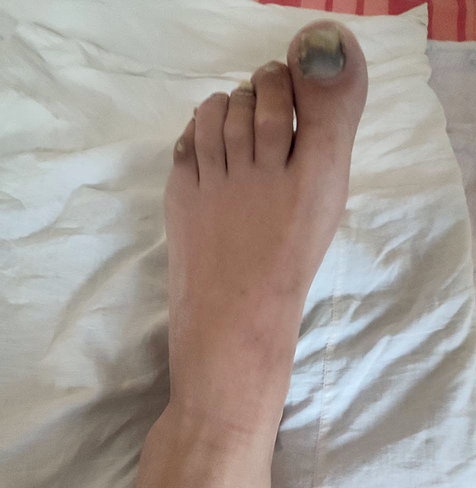
CASE REPORT. In January 2024, a 42-year-old male with secondto third-degree frostbite and threatened amputation of the left big toe was admitted to the burn unit at the V.K. Gusak Institute of Urgent and Recovery Surgery. Upon medical board consultation with specialty physicians and a clinical pharmacologist, the patient was prescribed off-label treatment with Neovasculgen. This was the first experience of using Neovasculgen in complex frostbite therapy of a patient in critical condition. The patient received periarterial injections of the medicinal product in the middle third of his left calf at a dose of 1.2 mg (at a 1:20 dilution with water for injection) on days 6 and 14 from the cold injury. The procedure was guided by Doppler ultrasonography of the calf vessels. After 2 days from the first injection, the patient experienced positive changes, including warming of the skin over the distal area of his left foot and a decrease in pain severity. After 5 days, the patient demonstrated a decrease in oedema and cyanosis of the toes, an increase in the active and passive range of toe motion, and an improvement in the supporting function of the foot. On day 15, the patient was discharged from the hospital in good condition. He had no pain or paraesthesia in his foot, and its weight-bearing ability and active and passive range of motion were fully restored.
CONCLUSION. This outcome offers a potential way to improve the medical care available to patients with cold injury: Neovasculgen integration into complex frostbite therapy provides an opportunity to shorten hospitalisation and rehabilitation periods and reduce the risk of disability. However, a systematic analysis and validation of clinical efficacy and safety data and a cost-benefit analysis are needed to confirm the feasibility of cold injury treatment with Neovasculgen.
INTRODUCTION. Human immunoglobulins have been successfully used in clinical practice to treat multiple autoimmune and inflammatory conditions, and most of the current immunoglobulins are well tolerated by patients. However, patients may develop complications associated with proteins and other components of human plasma present in immunoglobulin products. The improvement of measures to ensure the quality, efficacy, and safety of immunobiologicals, including intravenous immunoglobulins (IVIGs), requires regular monitoring of data on individual and class-related adverse drug reactions (ADRs) associated with these medicinal products.
AIM. This study aimed to conduct a systematic and comprehensive assessment of information on the potential ADRs to the IVIGs of Russian marketing authorisation holders from summaries of medicinal product characteristics (SmPCs) and the Russian national pharmacovigilance database.
MATERIALS AND METHODS. The study analysed the SmPCs of all the IVIGs authorised for use in Russia and the spontaneous reports of ADRs to the IVIGs of Russian marketing authorisation holders. The study was limited to the spontaneous reports submitted to the database PHARMACOVIGILANCE/MONITORING OF CLINICAL TRIALS OF MEDICINES in the Automated Information System of the Federal Service for Surveillance in Healthcare from 1 January 2020 to 30 August 2024.
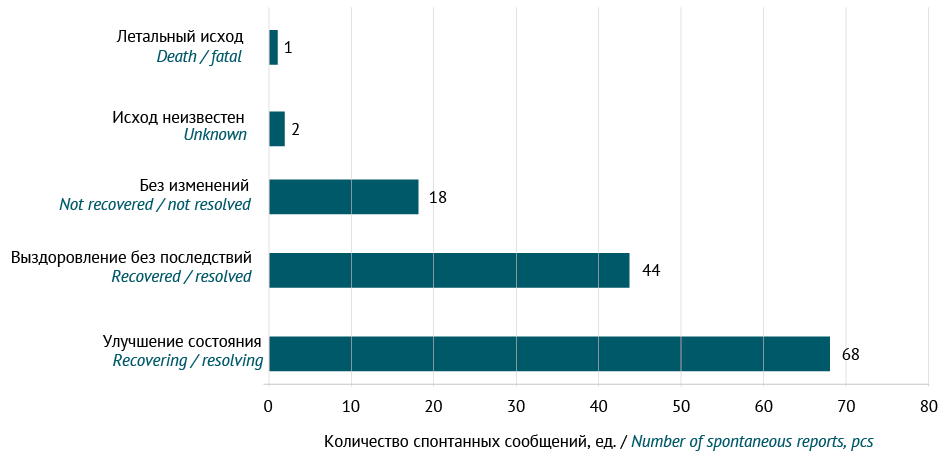
RESULTS. At the time of the study, there were 20 normal human IVIGs authorised in Russia, and Russian companies held marketing authorisations for 9 of these IVIGs. During the study period, there were 133 cases of ADRs associated with the IVIGs of Russian marketing authorisation holders. The most common ADRs included 41 (16.5%) cases of hyperthermia, 14 (5.6%) cases of headache, 14 (5.6%) cases of urticaria, and 14 (5.6%) cases of treatment ineffectiveness. The majority of these cases (70%) occurred when IVIGs were used for approved indications.
CONCLUSIONS. All the ADRs identified in the analysis of the Russian national pharmacovigilance database were expected and consistent with the ADRs labelled in the SmPCs of the reported IVIGs. To reduce the risk of ADRs and improve the safety of IVIG therapy, it is required to develop a comprehensive approach to human IVIGs with quality assurance measures and standard use guidelines.
INTRODUCTION. With the development of the Internet and the increasing availability of social networks and fora, patients have received an opportunity to share their medication experiences online. According to the guidelines on Good Pharmacovigilance Practices, social media can be considered an important additional source of patient-derived information in post-marketing surveillance, but the effectiveness of their use in detecting adverse drug reactions (ADRs) is still being investigated.
AIM. This study aimed to analyse the results of relevant original studies and assess the potential of using social networks and online patient fora as a source of information on ADRs associated with the use of medicinal products.
DISCUSSION. Published studies indicate that posts on social networks and patient fora describe both minor and serious ADRs, including new ADRs. The relevance of social media as a source of information about the safety of a medicinal product varies depending on several factors, including the medicinal product class and time on the market, as well as the platform demographics. Young users (18–44 years) are interested in online discussions about medicinal products for mental and reproductive system disorders. Users aged 45–64 years tend to discuss the use of medicinal products for chronic pain (including muscle pain), menopause, and gastritis. Discussions among users over 65 years old predominantly focus on medicinal products for diabetes, heart conditions, and muscle pain. People are much more likely to describe ADRs associated with the use of medicinal products for orphan diseases and cancer on fora for patients than on social networks in general, and vice versa for ADRs associated with the use of medicinal products for mental disorders. In addition, social media may be of interest as a source of information about cases of overdose, misuse and off-label use of medicinal products, and use of medicinal products during pregnancy and lactation.
CONCLUSIONS. Social media can be a source of valuable information about the safety of medicinal products and the impact of ADRs on the quality of patients’ lives. Marketing authorisation holders can obtain new information about the safety of medicinal products by extending their safety monitoring strategies to include social media. Nevertheless, since the relevance of a particular social network or patient forum for the detection of ADR cases varies considerably, a preliminary assessment is necessary to ascertain the presence of information on the medicinal product of interest.
PRECLINICAL STUDIES
INTRODUCTION. Developing novel medicines based on non-pathogenic enterovirus strains exhibiting oncotropic and oncolytic properties represents an up-to-date and safe approach to complex cancer treatment and postoperative metastasis prevention. Safety pharmacology studies are a necessary step in the preclinical development of medicinal products.
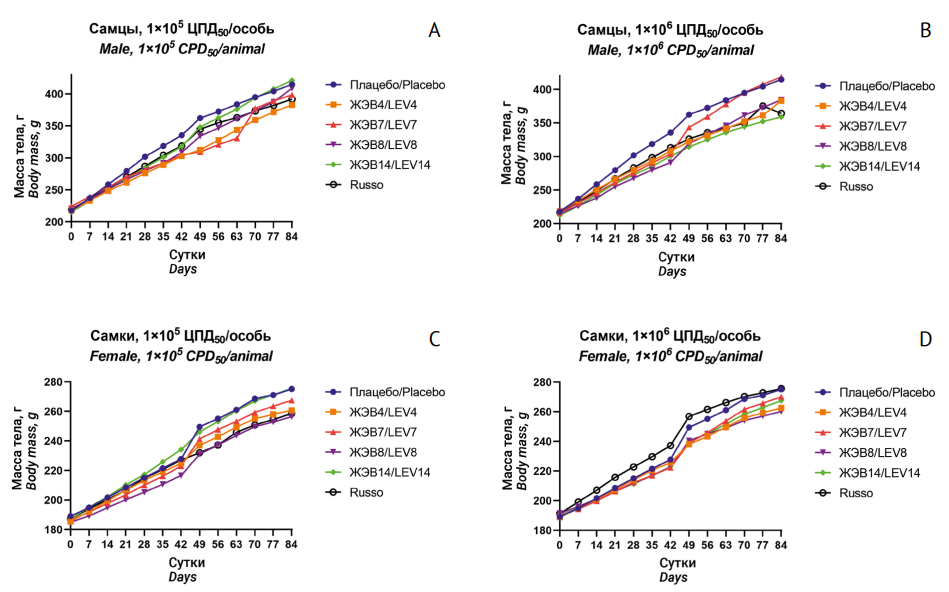
AIM. The study aimed to investigate the single and repeated-dose general toxicity, local tolerance, safety pharmacology, and pyrogenicity of medicinal products based on non-pathogenic LEV4, LEV7, LEV8, LEV14, and Russo enterovirus strains as part of preclinical safety studies.
MATERIALS AND METHODS. The study used medicinal products of highly purified group A, B, and C enteroviruses at a titre of 2×107–5×108 CPD50/mL (CPD50 is a cytopathogenic dose of the virus causing 50% cell lysis) and normal saline as a diluent. The viruses were propagated in Vero cells. The safety study used 220 male and female BALB/c mice, 440 male and female Wistar rats, and 18 male Soviet chinchilla rabbits. The study animals received an intravenous dose of 1×105 or 1×106 CPD50/animal once (single-dose toxicity) or weekly for 90 days (repeated-dose toxicity). Clinical examination, laboratory testing, and necropsy were performed on Days 45 and 91 of the experiment. Statistical data processing was performed using Prism 8.0 software (GraphPad Software, Inc., USA).
RESULTS. Upon single administration of each of the five enterovirus medicinal products to mice and rats, the authors observed complete survival, upward trends in body weight gain, and no gross or histopathological changes in the brain, spleen, liver, kidneys, lungs, or at the injection site. Upon repeated administration at the study doses, the medicinal products caused no functional changes in the organs and systems. All the studied parameters were within the normal physiological ranges for male and female rats. Histopathological examination revealed no pathological changes or specific cytolytic and/or cytopathic effects. No local irritation was observed. None of the investigational medicinal products showed pyrogenicity.
CONCLUSIONS. The obtained preclinical results demonstrate the safety of antineoplastic agents based on live non-pathogenic LEV4, LEV7, LEV8, LEV14, and Russo enteroviruses.
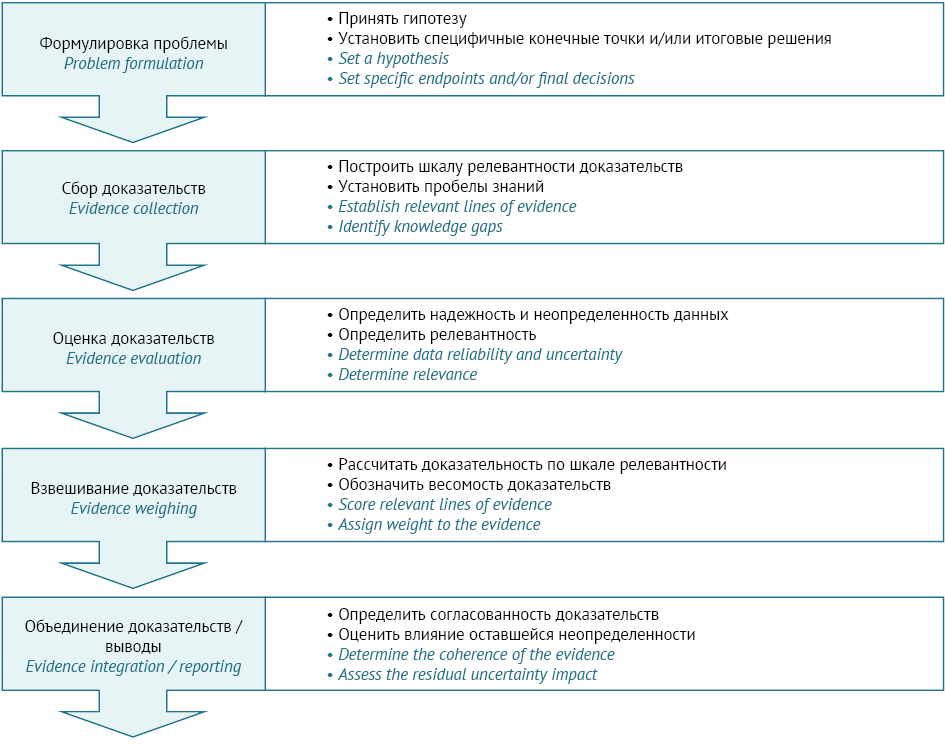
INTRODUCTION. In recent years, the Russian Federation and the Eurasian Economic Union have included the concept of ‘weight of evidence’ (WoE) in regulatory documents. International regulators place great emphasis on data transparency in documenting and assessing the WoE, and their position is reflected in the relevant regulatory documents. However, the Russian Federation has faced the absence of consistent Russian-language terminology in this area, the lack of a clear vision of WoE assessment principles, and the need for a sound understanding of applied WoE methods for pharmaceutical development and regulatory review of preclinical study results. These gaps require collated information on WoE assessment and practical guidance for its use.
AIM. This study aimed to analyse regulatory documents as well as scientific and methodological publications on the WoE concept and assessment methods in order to investigate the opportunities and practical applications of WoE analysis in the development of medicines and the regulatory review of study results.
DISCUSSION. This article covers documents by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), the Organisation for Economic Co-operation and Development (OECD), the European Chemicals Agency (ECHA), and other organisations. Using these documents and taking into account Russian terminology, the authors clarify the WoE as a concept and explain its assessment. Furthermore, the article classifies the key risk factors that must be considered in WoE analysis, with examples drawn from immunotoxicity, reproductive toxicity, and carcinogenicity studies. Analysing such data is critical to the design of preclinical studies, interpretation of their results, and safety evaluation of medicines. This article provides examples of WoE assessments conducted to consider the need for additional preclinical studies in juvenile animals in the development of small molecules and monoclonal antibodies for paediatric use.
CONCLUSION. The WoE assessment algorithms and criteria described in this article may be implemented by preclinical study initiators, their research teams, and regulatory experts evaluating medicines.
ERRATUM
COCHRANE PUBLICATIONS
This article is a Russian translation of a Plain Language Summary (PLS) of a Cochrane Review previously published in the Cochrane Database of Systematic Reviews. Original publication: Shalviri G, Mohebbi N, Mirbaha F, Majdzadeh R, Yazdizadeh B, Gholami K, Grobler L, Rose CJ, Chin WY. Improving adverse drug event reporting by healthcare professionals. Cochrane Database Syst Rev. 2024;(10): CD012594. https://doi.org/10.1002/14651858.CD012594.pub2
ISSN 2619-1164 (Online)