AUTHORITATIVE OPINION
The origins of the journal Safety and Risk of Pharmacotherapy date back to 1994. The journal was published as Safety of Medicinal Products, Safety of Medicines, and Safety of Medicinal Products and Pharmacovigilance. In Russia, this journal has always been the only specialised scientific publication covering exclusively the safety of pharmacotherapy. Alla V. Astakhova, Candidate of Medical Sciences, headed the journal from 1994 to 2015.
The history of the journal is closely intertwined with the history of the pharmacovigilance system in Russia. Vladimir K. Lepakhin, Doctor of Medical Sciences, Professor, Corresponding Member of the Russian Academy of Sciences, has made an outstanding contribution to the development of the Russian pharmacovigilance system. This joint interview with Alla V. Astakhova and Vladimir K. Lepakhin presents the major milestones in the development of the journal and the Russian pharmacovigilance system.
MAIN TOPIC: CURRENT ISSUES IN PHARMACOVIGILANCE
SCIENTIFIC RELEVANCE. Inconsistent terminology for vaccine safety may create challenges for healthcare professionals in identifying, interpreting, and assessing adverse events following vaccination in clinical practice.
AIM. The authors aimed to review Russian and international terminology for adverse events following vaccination.
DISCUSSION. This review covers the terminology used in Russian, EAEU, WHO, and ICH documents. The term “adverse event” is used in most Russian and international pharmacovigilance regulations and guidelines; this term universally defines undesirable consequences associated with medicinal products regardless of their therapeutic class. Russian regulations and guidelines use various terms to describe adverse events following vaccination, including “adverse events”, “side effects of vaccines”, and “post-vaccination complications”. In 2019, the Ministry of Health of Russia approved the Guidelines for the Detection, Investigation, and Prevention of Side Effects Following Immunisation. However, the term “side effects following immunisation” cannot unambiguously characterise the safety of vaccines, as this term can refer to negative, neutral, or positive events following vaccination. The term “post-vaccination complications” is defined in Russian legislation as a list of pathological conditions. The list is not exhaustive and does not fully reflect the definition of post-vaccination complications as severe and/or persistent health issues following vaccination. The term “adverse events following immunisation”, which is recommended by most international guidelines, describes the negative consequences of vaccination more accurately. At the same time, the term “post-vaccination complications” can be reformulated as “serious adverse events following vaccination” with clearly defined and categorised criteria.
CONCLUSIONS. In addition to aiding in detecting, classifying, and evaluating adverse events following immunisation, the unification of terminology for vaccine safety in Russian regulations and guidelines will also facilitate risk mitigation in both individual and mass vaccination campaigns in general.
SCIENTIFIC RELEVANCE. The safety assessment of investigational medicinal products is a mandatory step in clinical trials of all phases, including bioequivalence studies. However, there are no approaches providing for the individualised assessment of adverse drug reactions (ADRs), which contributes to the quality of decisions on the safety of pharmacotherapy.
AIM. The study aimed to develop and justify approaches to the individualised assessment of the safety of pharmacotherapy based on quantitative integrative analysis of adverse events (AEs).
MATERIALS AND METHODS. The authors carried out a systematic review of open-access publications and adapted quantitative integrative analysis methods for assessing the safety of pharmacotherapy in clinical trials involving healthy volunteers. The developed methodology is a step-by-step individualised assessment of ADRs, where each case is assigned a certain score and a weight, with subsequent data aggregation to obtain an integrated indicator at the system/organ and organism levels.
RESULTS. The authors developed a five-step procedure for assessing the safety of pharmacotherapy based on quantitative integrative ADR analysis. This procedure involves scoring an AE, converting the score using membership functions, assigning weights, aggregating data to obtain an integrated indicator, and interpreting individual and group indicators. The sequential implementation of the analysis steps in accordance with the proposed procedure makes it possible to assign each volunteer (study subject) to a specific group in accordance with the likelihood of developing AEs. In addition to individual assessment, the article presents an algorithm for interpreting indicators for groups of study subjects, depending on the treatment group (study or comparator medicinal product).
CONCLUSIONS. The described algorithm for converting and presenting integrative AE assessments will improve the reliability and validity of conclusions on the safety of medicinal products, which is important for planning and implementing further clinical development programmes.
SCIENTIFIC RELEVANCE. Pain and pain relief are among the most important problems that arise during medical procedures. Lidocaine is used not only as an anaesthetic during interventions but also as a diluent for other medicinal products. Analysis of adverse drug reactions (ADRs) associated with lidocaine contributes to studying lidocaine toxicity and, as a result, developing measures to reduce side effects.
AIM. The authors aimed to conduct a retrospective analysis of spontaneous reports of fatal ADRs associated with lidocaine.
MATERIALS AND METHODS. This study analysed spontaneous ADR reports of fatal outcomes associated with lidocaine submitted to the federal ADR database in the Automated Information System of the Federal Service for Surveillance in Healthcare of the Russian Federation (Pharmacovigilance, versions 1.0 and 2.0) from 1 January 2008 to 31 December 2020.
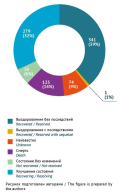
RESULTS. The ADR reports included 102 fatal outcomes associated with lidocaine. Most often, lidocaine was prescribed as a local anaesthetic or as a diluent for antibacterials. Studying the administered lidocaine doses, the authors identified several overdose cases. Most life-threatening conditions were due to hypersensitivity reactions of anaphylactic shock (54 cases, 52.9%). Lidocaine administration was accompanied by loss of consciousness, respiratory failure, and cardiac arrest in 10 cases (9.8%). Convulsions were a significant clinical sign in 9 cases (8.8%).
CONCLUSIONS. Awareness of the risks of systemic toxic effects of local anaesthetics, including lidocaine, necessitates the practical implementation of certain safety measures, such as allergy skin tests before administration and mandatory monitoring of doses and patients’ well-being after administration.
SCIENTIFIC RELEVANCE. The pandemic of novel coronavirus infection (COVID-19) led to a drastic increase in the use of medicinal products of various therapeutic groups and increased spontaneous reporting of adverse drug reactions (ADRs). Therefore, it is necessary to analyse the reported information to identify potential safety signals.
AIM. This study aimed at systematisation and quantitative analysis of data on the safety of COVID-19 medicinal products from the Russian pharmacovigilance database.
MATERIALS AND METHODS. This retrospective analysis included spontaneous ADR reports submitted to the Russian pharmacovigilance database from 1 January 2020 to 31 December 2022. The authors applied disproportionality analysis to generate safety signals.
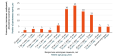
RESULTS. During the stated period, the database website published 873 spontaneous reports on 1,636 ADRs associated with COVID-19 treatment. Most ADRs were associated with favipiravir (493 reports), hydroxychloroquine (87 reports), and olokizumab (85 reports). The most common ADRs included 273 (16.7%) abnormal investigation results, 203 (12.4%) hepatobiliary disorders, and 191 (11.6%) gastrointestinal disorders. The majority of adverse events, 674 (77%) cases, had favourable outcomes. Using frequency-based disproportionality analysis, the authors identified 23 potential safety signals based on disproportionately reported ADRs for azithromycin, dexamethasone, levilimab, lopinavir+ritonavir, molnupiravir, olokizumab, tofacitinib, tocilizumab, umifenovir, and favipiravir.
CONCLUSIONS. The new safety signals require additional assessment of ADR reporting forms for causal relationship analysis, validation, prioritisation, and clinical interpretation. The frequency-based method did not identify safety signals for hydroxychloroquine, remdesivir, baricitinib, sarilumab, and anti-COVID-19 human immunoglobulin, but this does not rule out the possibility of detecting new causal relationships.
SCIENTIFIC RELEVANCE. The clinical features of COVID-19 in patients with comorbidities, including diabetes mellitus (DM), have already been discussed in the medical literature. However, the available data on blood glucose levels in patients with DM during SARS-CoV-2 infection and after COVID-19 vaccination are clearly insufficient to estimate the importance of the changes taking place.
AIM. The study aimed to show that patients with DM or impaired glucose metabolism need glycaemic monitoring during COVID-19 disease and after COVID-19 vaccination, drawing on the example of a clinical case.
MATERIALS AND METHODS. The study analysed the medical records of a 58-year-old male patient newly diagnosed with DM. He received inpatient and outpatient treatment after COVID-19 vaccination and SARS-CoV-2 infection in February–November 2021. In 2014, the patient was diagnosed with impaired glucose metabolism, including fasting hyperglycaemia (≤ 9 mmol/L), which was corrected by diet.
RESULTS. After vaccination with Gam-COVID-Vac component 1 in February 2021, the patient developed polydipsia, polyuria, and arterial hypertension. His laboratory findings were as follows: blood glucose, 25 mmol/L; glycated haemoglobin, 10.7%; fasting insulin, 28.4 μIU/mL; calcium, 2.45 mmol/L; and 25-hydroxyvitamin D, 21 ng/mL. The patient was diagnosed with new-onset type 2 DM, admitted to the endocrinology department of a multidisciplinary hospital, and discharged when his condition stabilised after 14 days of treatment. After vaccination with Gam-COVID-Vac component 2, the patient’s glucose levels did not change. In November 2021, the patient was diagnosed with SARS-CoV-2 infection. Even though all symptoms had resolved within 3 days, the virus persisted in the blood for 12 days without clinical manifestations of the disease. This was confirmed by repeated polymerase chain reaction testing. The patient had moderate hyperglycaemia despite antidiabetic treatment; his glucose levels were restored to normal without hospitalisation.
CONCLUSIONS. Timely vaccination against COVID-19 in patients with DM, hypertension, and obesity contributes to a mild course of COVID-19 and helps avoid complications in the lungs and other organs. For patients with DM or glucose metabolism disorders, blood glucose monitoring is advisable for detecting and correcting possible hyperglycaemia after vaccination and/or recovery from COVID-19.
PRECLINICAL STUDIES
SCIENTIFIC RELEVANCE. The Tox21 (Toxicology in the 21st Century) programme was developed by the US Tox21 Consortium with the aim to replace animal-based toxicity assessments of chemicals with a wide range of in vitro and in silico testing approaches and has since been successfully applied in practice.
AIM. The study aimed to review information on alternative in vitro models developed as part of the Tox21 programme for testing the toxicity of chemical compounds.
DISCUSSION. According to the information provided by the National Toxicology Program, Environmental Protection Agency, National Center for Advancing Translational Sciences, and other Tox21 Consortium members on their official websites and in the literature, the Tox21 Consortium has developed a quantitative high-throughput screening technology for testing the safety of chemicals and created the Tox21 10K library of chemical compounds using this screening technology. The library has been successfully used to create models that predict the toxicity of chemicals prior to preclinical studies. Researchers have proposed new approaches to studying the safety of chemical compounds in human cell lines to replace in vivo studies. Innovative organ-on-chip, multi-organ-on-chip, and organoid models are free from the drawbacks and limitations of cell-line models and offer more accurate representations of complex cell–matrix and organ–organ interactions. Developed under the Tox21 programme to search for new chemical toxicity biomarkers and gene signatures, novel transcriptomics (toxicogenomics) technologies can be used to classify toxicants according to their health risks and to identify potential side effects long before discovering any pathological changes in the body. The Interagency Coordinating Committee on the Validation of Alternative Methods conducts technical evaluation of alternative testing methods and promotes their implementation into regulatory practice.
CONCLUSIONS. Thus, new tools and technologies provide an opportunity for switching from in vivo toxicity testing of candidate medicinal products to in silico and in vitro methods.
SCIENTIFIC RELEVANCE. The high prevalence of fungal skin infections motivates expanding the range of sertaconazole products for external use.
AIM. The study was a preclinical comparison of the safety, antifungal activity, and pharmacokinetics of Sertaverin® 2% medicated shampoo (VERTEX JSC, Russia) with those of Sertamicol® 2% solution for external use (Glenmark Pharmaceuticals Ltd, India) and Nizoral® 2% shampoo (Janssen Pharmaceuticals N.V., Belgium) approved in the Russian Federation.
MATERIALS AND METHODS. In the toxicity study, the medicinal products were applied to the skin of male and female outbred rats at doses of 0.5 or 1.5 mL/animal for 28 days. The authors evaluated the pharmacokinetics of two sertaconazole formulations (shampoo and solution) following a single administration to adult male rats at the same dose. Nizoral® was not used in the pharmacokinetics study because it contains a different active substance, ketoconazole. The minimum inhibitory concentration (MIC) was determined using the serial microdilution method in a wide range of concentrations.
RESULTS. The medicinal products did not exhibit any significant toxic effects in laboratory animals after 28 days of repeated dermal application. Plasma sertaconazole concentrations were negligible. Sertaconazole was intensively distributed in the liver, which is a highly vascularised organ, and in the target organ (skin at the site of application). The relative bioavailability of sertaconazole from the shampoo relative to that from the solution for external use was approximately 30% in liver tissues and approximately 363% in skin tissues at the application site. Sertaverin® was comparable to sertaconazole in the active substance form in terms of inhibiting the growth of Malassezia furfur strains. The MICs calculated on the active substance basis were ≤16–64 μg/mL.
CONCLUSIONS. With its synergistic dual mechanism of action, broad-spectrum antifungal activity, lipophilic properties, and low systemic absorption, Sertaverin® may provide a more effective and safe alternative to marketed medicinal products for scalp diseases.
SCIENTIFIC RELEVANCE. Currently, there are no effective and safe medicinal products for idiopathic male infertility. Previous studies in two animal models of infertility (short-term cryptorchidism in rats and doxorubicin-induced testicular injury in mice) have shown the effectiveness of an originator medicinal product based on the mesenchymal stromal cell (MSC) secretome.
AIM. The aim of the study was to evaluate the toxicity profile of the MSC secretome-based medicinal product in rats after local intratesticular or intramuscular administration.
MATERIALS AND METHODS. The MSC secretome is a combination of factors secreted by MSCs in low-glucose Dulbecco’s modified Eagle’s medium (DMEM-LG) for MSC conditioning. In the single-dose toxicity study, the MSC secretome-based medicinal product was injected under the testicular tunica albuginea of male Wistar rats (15 per group) at doses of 15 and 25 relative units (RU) per animal, which are 1.5 and 2.5 times higher than the therapeutic dose (10 RU). In the repeat-dose toxicity study, male Wistar rats (10 per group) received intramuscular thigh injections of the medicinal product on days 1, 6, and 12 at doses of 15 and 25 RU per animal. The local tolerance study involved histopathological examination of the testes and thighs at the injection site. All studies included control groups of intact animals and animals similarly injected with blank DMEM-LG. The early follow-up period was 14 days, and the late follow-up period was 42 days.
RESULTS. The rats showed no changes in the general condition after single and repeated doses of the MSC secretome-based medicinal product. Single subtunical doses induced moderate irritation; its signs included pathological changes in individual seminiferous tubules: epithelial atrophy (70% of the animals on day 14; 55% at late follow-up) and sperm stasis (70% of the animals). Similar changes were observed in the blank DMEM-LG group (up to 80% of the animals). There were no pathological changes in the tissues after repeated injections. A transient increase in alkaline phosphatase activity was detected in animals after their third intramuscular injection at a dose of 25 RU; the other biochemical parameters were normal in all study groups.
CONCLUSIONS. The MSC secretome-based medicinal product has a favourable safety profile following both intratesticular and intramuscular administration, as it does not cause any permanent changes in the studied organs and tissues.
RELEVANT INFORMATION
The experts of the Department for Medicine Safety Evaluation of the Scientific Centre for Expert Evaluation of Medicinal Products analysed administrative decisions of international pharmacovigilance regulatory authorities on the necessary labelling updates. The analysis revealed 16 decisions containing safety updates for the following medicines registered in Russia: avatrombopag, amphotericin B, acetazolamide, belimumab, bendamustine, hydrocortisone, dabrafenib, dexamethasone, denosumab, ibuprofen, cortisone, prednisolone, trametinib, nilotinib, pirfenidone, and cefotaxime.
ISSN 2619-1164 (Online)