MAIN TOPIC: PATIENT SAFETY: THE WAY PRECLINICAL DATA AND POST-AUTHORISATION PHARMACOVIGILANCE SHAPE MODERN PHARMACOTHERAPEUTIC LANDSCAPE
INTRODUCTION. Development of safe and efficacious radiopharmaceutical medicines (radiopharmaceuticals) requires a legally, organisationally and scientifically sound regulatory system. Due to the lack of current Russian and Eurasian Economic Union (EAEU) guidelines for preclinical development of radiopharmaceuticals, international approaches to the scope of preclinical research and expert review of the obtained results.
AIM. The study aimed to update expert approaches to preclinical studies of modern radiopharmaceuticals by analysing the relevant scientific recommendations, current regulatory requirements, and expertise in Russia and abroad.
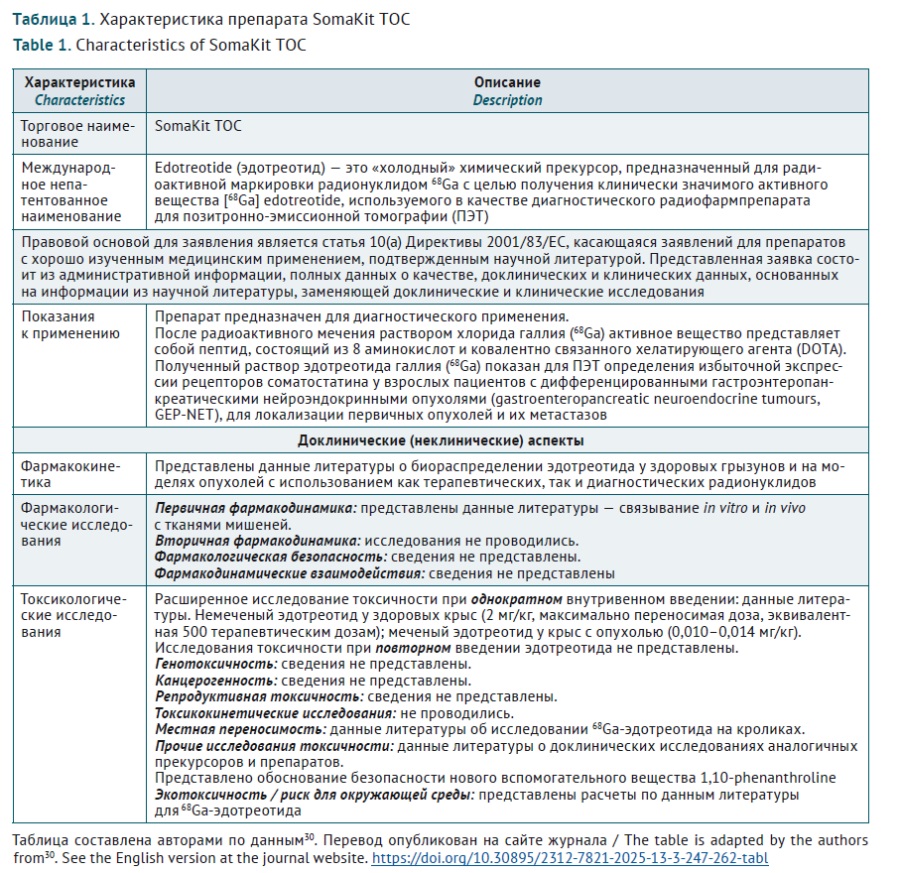
DISCUSSION. In general, radiopharmaceuticals are considered as medicinal products in most countries. To introduce an originator product on the market, a full development cycle is required, including assessment of specific activity, toxic properties, pharmacokinetic and pharmacodynamic parameters. General principles of preclinical studies set out in the relevant EAEU guidelines are applicable to research of radiopharmaceuticals, with radioactivity being a special property that requires consideration. Specific preclinical studies of targeted radiopharmaceuticals include: defining pharmacological target; selecting and developing targeting vectors for radionuclide delivery; selecting chelators for coupling radionuclides to vectors; choosing a radionuclide; assessing in vitro binding specificity and activity; assessing cytotoxicity; selecting a relevant in vivo model for assessing biodistribution and therapeutic efficacy or imaging; dosimetry; toxicological studies; and preparing for translation into clinical trials. General form of the European Union’s non-clinical assessment report of preclinical mostly coincides with the EAEU expert report on preclinical (non-clinical) study results.
CONCLUSIONS. The approach to conducting preclinical studies of radiopharmaceuticals and assessing their results is fundamentally similar in the EAEU and the European Union. Basic regulatory and expert requirements can be defined, alongside with the general recommendations for preclinical development of radiopharmaceuticals. Together with common methodological recommendations, it is reasonable to compile appendices for certain radiopharmaceutical groups based on current scientific data and regulatory experience in the Russian Federation and abroad.
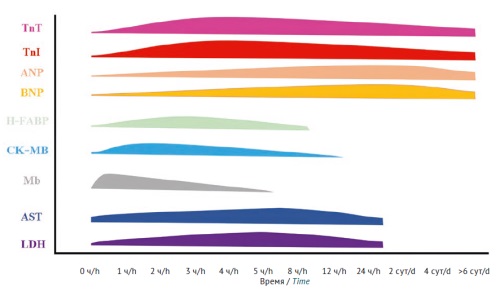
INTRODUCTION. The cardiovascular safety evaluation of medicines using in vivo models is a necessary preclinical step that is performed either in safety pharmacology studies or in toxicity studies. The design of safety pharmacology studies primarily involves assessing the potential of a test substance to prolong cardiac ventricular repolarisation, without in-depth investigation of potential structural damage to the heart and blood vessels. Toxicity studies usually do not include electrophysiological testing. The regulatory standards of the Eurasian Economic Union (EAEU) and the International Council for Harmonisation (ICH) lack detailed guidance on the use of specific markers of cardiovascular dysfunction.
AIM. This study aimed to develop an integrated approach to assessing the cardiac and vascular toxicity of medicinal products in preclinical in vivo studies.
DISCUSSION. Cardiovascular function can be assessed in both small laboratory animals (rodents) and larger animals, such as rabbits, ferrets, dogs, minipigs, and primates. The toxic effects of a test medicinal product on the cardiovascular system of animals may be manifested as physiological, biochemical, and structural changes in the systems and organs. Therefore, the assessment of cardiovascular function should be based on a combination of instrumental, laboratory, and histological methods. First of all, physiological and laboratory studies are applicable. It is recommended to perform electrocardiography, heart rate and blood pressure measurements, and quantification of markers of cardiovascular dysfunction and structural cell damage. For more in-depth analysis, histological and immunohistochemical studies of cardiac and vascular tissues are recommended to assess changes at the tissue and cellular levels.
CONCLUSIONS. An effective strategy for detecting cardiovascular disorders is the use of an integrated approach that, on the one hand, facilitates a comprehensive assessment of the possible toxic effects of a medicinal product and, on the other hand, increases the translational potential of the data obtained at the preclinical stage of research.
INTRODUCTION. Hepatotoxicity is the leading cause of isoniazid discontinuation, significantly reducing the efficacy of antituberculosis therapy, increasing the risk of disease relapse, and contributing to secondary drug resistance in Mycobacterium tuberculosis. The development of hepatotoxic reactions to isoniazid is associated with genetic variations in the activity of N-acetyltransferase 2 (NAT2), an enzyme responsible for hepatic biotransformation of the medicinal product. Dose reduction may prevent liver damage in slow acetylators. However, there is no unified algorithm for dosing this antituberculosis medicinal product based on the results of pharmacogenetic testing.
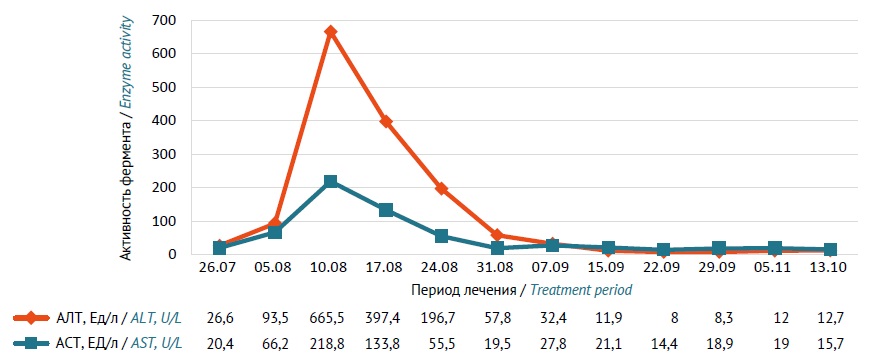
CASE DESCRIPTION. A 35-year-old man, a Yakut, was diagnosed with A16.0 Focal tuberculosis in the upper lobes of both lungs (S1–2) at the stage of infiltration without isolation of Mycobacterium tuberculosis in 2022 and was treated in the respiratory tuberculosis department of the E.N. Andreev Phthisiology Research-Practice Center, Republic of Sakha (Yakutia). Diagnosis was confirmed via clinical, laboratory, and imaging findings. Initial therapy included a daily oral combination comprising isoniazid (500 mg), rifampicin (600 mg), pyrazinamide (1750 mg), and ethambutol (1200 mg). During the intensive of therapy, the patient developed moderate hepatocellular liver damage, which manifested as nausea, vomiting (one episode), weakness, and epigastric pain. Serum alanine transaminase (ALT) increased to 665.5 U/L and aspartate transaminase (AST) increased to 218.8 U/L. Ultrasound examination revealed hepatomegaly, diffuse hepatic and splenic parenchymal changes, chronic cholecystitis, and reactive pancreatitis. Pharmacogenetic testing identified NAT2 allelic variants (*5, *11, *12) associated with slow isoniazid acetylation. When treatment continued, the dose of isoniazid was reduced to 300 mg/day; the patient took the other medicines at the same doses. The tolerability of antituberculosis therapy was satisfactory, and hepatotoxic reactions did not develop. The intensive phase of tuberculosis treatment was 64 days. Showing clinical and radiological improvement, the patient was discharged from the hospital. Therapy in the continuation phase included a combination of isoniazid (300 mg) and rifampicin (600 mg) once a day for 120 days. After completion of therapy, the patient was considered clinically cured and was transferred to group III of dispensary observation (regular follow-up visits).
CONCLUSIONS. Individualisation of isoniazid dosing according to the results of pharmacogenetic testing allowed the medical team to continue treatment of the patient with drug-sensitive pulmonary tuberculosis and to avoid recurrent development of isoniazid-induced liver damage.
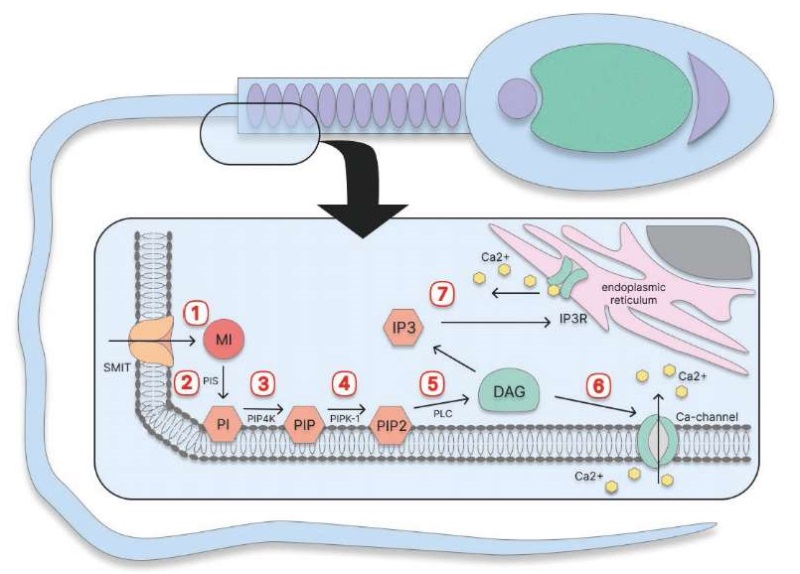
INTRODUCTION. Male infertility, in particular reproductive system deficiency, is often associated with oxidative stress. Myo-inositol antioxidant properties are essential for various biochemical and cellular processes in spermatozoa; for example, they contribute to mitochondrial function and sperm motility. This creates an opportunity to use inositol in the complex infertility treatment of various aetiology, including oligoasthenoteratozoospermia, asthenozoospermia, and in assisted reproductive technologies.
AIM. This study aimed to evaluate the possibility and effectiveness of using inositol for spermatogenesis restoration in male reproductive dysfunction caused by oxidative stress.
DISCUSSION. Oxidative stress damages sperm cell membrane, causes mitochondrial dysfunction, and affects DNA. Studies show that myo-inositol positively impacts spermatogenesis due to its antioxidant properties and activation of mitochondrial metabolism. It promotes ATP production, reduces oxidative damage markers, decreases DNA fragmentation and improves sperm morphology. Moreover, myo-inositol increases cellular testosterone, optimises actin — globulin binding, thus enhancing sperm motility, and modulates intracellular calcium concentrations necessary for oxidative metabolism and ATP synthesis. Clinical studies have shown that inositol increases overall sperm motility by 20–30%; progressive motility — up to 40%; fertilisation rate — by 10–15%, and reduces DNA fragmentation by 3–5%. Myo-inositol used for sperm cryopreservation reduces reactive oxygen and apoptosis, maintains mitochondrial membrane potential, and increases oxidative phosphorylation efficiency by 6–15%.
CONCLUSIONS. Myo-inositol is a promising therapeutic compound for treating male infertility of various aetiology, including oligoasthenoteratozoospermia, asthenozoospermia, and for preparing sperm in assisted reproductive technologies. Also, due to its antioxidant properties, myo-inositol can be recommended in suspected or confirmed oxidative damage to sperm. However, large controlled trials are needed to confirm positive effect of myo-inositol on male reproductive function.
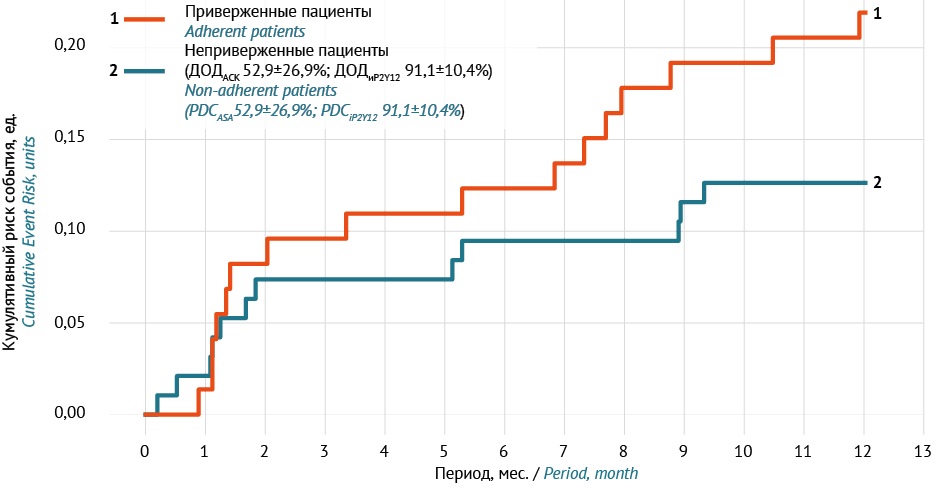
INTRODUCTION. Actual effectiveness and safety of dual antiplatelet therapy (DAPT) can be evaluated only when patients genuinely adhere to prescribed therapy. Investigating the causes of non-adherence to DAPT inevitably involves a comprehensive analysis of adverse drug reactions (ADR), their clinical management, and the occurrence of clinically significant ischemic events in patients surviving acute myocardial infarction (AMI).
AIM. This study aimed to analyse the adherence dynamics to DAPT in the context of haemorrhagic complications, their pharmacological management, and clinically significant cardiac events in patients over the first year following AMI. The data were provided by Unified Medical Information Analysis System (EMIAS), Moscow, for the years 2021-2023.
MATERIALS AND METHODS. A retrospective analysis was performed using EMIAS data on patients who were under outpatient follow-up by cardiologists at a Moscow polyclinic for one year following AMI. "Method based on assessment of all medical records" (WHO) was used to register ADRs. Patient medication adherence was evaluated by tracking prescripton claims for individual DAPT components and in total. Only patients who demonstrated adherence to DAPT during the first six months of therapy (n=168) were included in the analysis.
RESULTS. Upon hospital discharge, patients received acetylsalicylic acid 100 mg (97.6%) in combination with a P2Y12 platelet inhibitor, predominantly ticagrelor (76.2%). During the second six-month period, 73 (44.5%) patients lost adherence to DAPT (non-adherent). Haemorrhages of any severity were recorded in 24.4% of patients over the year (total ADRs — 57); and in 15.5% in the first six months. Within 6–12 months, compared to non-adherent patients, the severity of bleeding according to the BARC scale was higher among those who maintained adherence (p=0.035), with serious haemorrhagic ADRs observed only in this group. DAPT adjustment by cardiologists was performed in 29.3% of patients with bleeding, more often in the first half of the year than in the second (22% vs 7.3%; p=0.029). The number of hospitalisations for cardiac reasons in 6–12 months was higher in the non-adherent group (p=0.047), who mainly discontinued acetylsalicylic acid (PDC 52.9±26.9%) for an average of 111.7±37.7 days (“therapy interruption”).
CONCLUSIONS. Among patients initially adherent to DAPT in the first six months, only 56.5% maintained adherence in the second half of the year following AMI. The annual incidence of haemorrhagic ADRs was 24.4%. These ADRs were more severe among those who maintained adherence (p=0.035), however, more non-adherent patients were hospitalised for cardiac reasons (p=0.047). Benefit-risk balance of haemorrhages and thrombosis should be monitored for long-term DAPT; personalised approach is feasible in clinical practice, including risk stratification of haemorrhages using PRECISE-DAPT/BARC scales; high-risk patients require early switch to monotherapy (such as clopidogrel after 3–6 months) focusing on the balance between anti-ischaemic effectiveness and risk of haemorrhage.
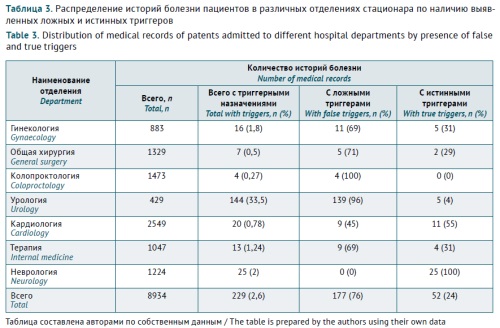
INTRODUCTION. Allergic drug reactions in hospitalised patients limit the opportunities for rational pharmacotherapy and increase the risk of polypharmacy due to the need for managing the patient’s condition and prescribing anti-allergic agents. An objective assessment of the prevalence of inpatient allergic drug reactions and a categorisation of medicinal products are critical for treatment adjustment and will lead to both a significant improvement in clinical outcomes for patients and a reduction in the financial burden for the healthcare system. The Global Trigger Tool (GTT) methodology is based on analysing medical records and capturing specific triggers, which makes the GTT easily applicable in clinical practice.
AIM. This study aimed to investigate the applicability of the GTT in studying the prevalence of allergic drug reactions in patients admitted to a multidisciplinary hospital.
MATERIALS AND METHODS. This study used the GTT in retrospective pharmacoepidemiological analysis of medical records of patients admitted to City Clinical Hospital 24 of the Moscow City Health Department from 1 October 2022 to 1 April 2023. The study included medical records of patients treated in the internal medicine and surgery departments during the specified period and excluded those of allergology patients.
RESULTS. A total of 8,934 patients were admitted to the internal medicine and surgery departments during the analysed period. Triggers suggestive of allergic drug reactions were identified in 229 (2.6%) of their medical records. This would correspond to a prevalence of 2,563 cases per 100,000 patients. However, the analysis of prescriptions, diary cards, and clinical and laboratory findings identified only 52 (22.7%) true triggers of allergic drug reactions. In the remaining 177 (77.3%) cases, the triggers were classified as false positives, as anti-allergic agents were prescribed before or concomitantly with the suspected medicinal product, presumably, to prevent potential allergic reactions. The main groups of medicinal products suspected to cause allergic reactions were systemic antimicrobial agents (22 (40.7%) products, in particular, 14 (20.3%) beta-lactam antibiotics) and monoclonal antibodies (21 (38.9%) products).
CONCLUSIONS. The true prevalence of allergic drug reactions was 0.58%, which corresponds to 582 cases per 100,000 patients. The study demonstrated the effectiveness of the GTT in identifying allergic drug reactions in real-world clinical practice. The exclusion of false triggers, first of all, anti-allergic agents prescribed as prophylaxis, significantly reduces the bias in estimating the true prevalence of allergic drug reactions and the risk of overdiagnosis.
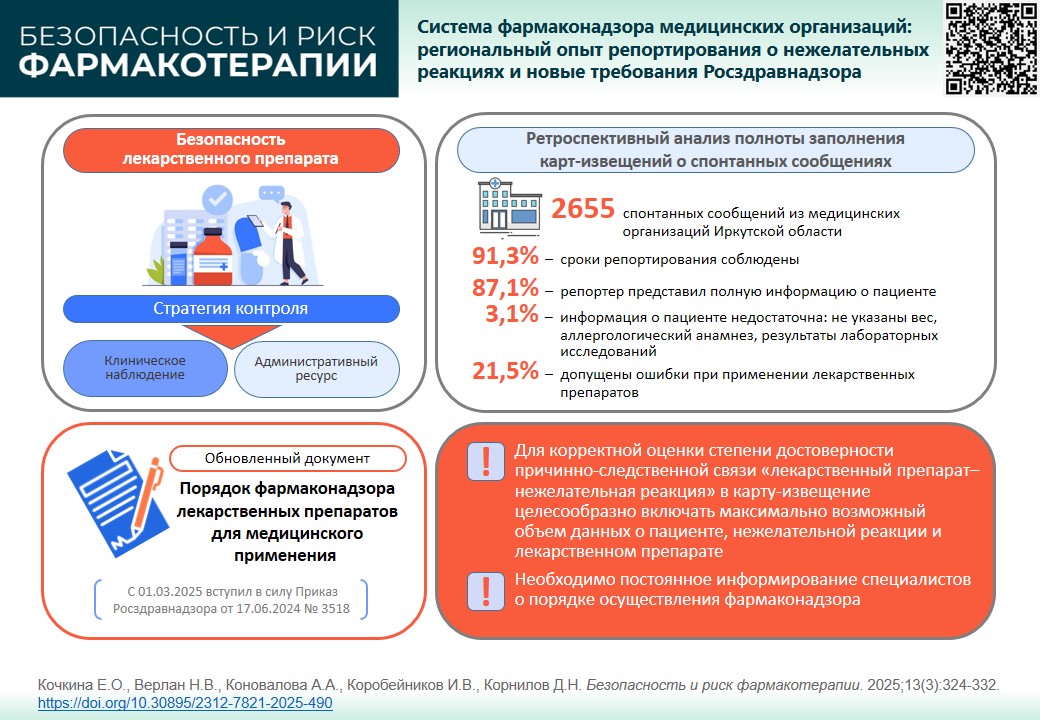
INTODUCTION. On 1 March 2025, an updated Pharmacovigilance procedure of medicinal products came into force complying with order of Roszdravnadzor No. 3518 as of 17 June 2024. Analysing reports submitted to Roszdravnadzor is a method that allows to adapt pharmacovigilance of a healthcare facility to the new requirements.
AIM. This study aimed to assess completeness and accuracy of reporting adverse drug reactions (ADR) to Roszdravnadzor. These data are essential to draft recommendations for filling out “Adverse drug reaction / no effect” form.
MATERIALS AND METHODS. The authors performed a post-hoc analysis of ADR reporting forms over 2009–2019 taken from Irkutsk region Safety monitoring centre and local data from Roszdravnadzor Automated Information System (2019–2023). Naranjo scale was used to assess the accuracy of the causal relationship between ADRs and medicinal product.
RESULTS. During the study period, medical facilities of Irkutsk region have submitted 2,655 spontaneous reports. The reporting deadlines were met in 91.3% of the cases. The ADR summary fully described the patient and medicinal products in 87.1% of the cases. Of the 2,655 ADRs, 72.3% described the underlying disease. The most common mistake (3.1%) was incomplete patient information (weight, allergic reactions, and laboratory findings). Lack of therapeutical effect was described in 2.0% of the cases. Drug misuse was reported in 21.5% of spontaneous reports (mostly prescribing antibacterials for viral infections). Causal relationship between the ADR and medicinal products was found in most of the cases (94.5%), the accuracy being classified as certain (25.0%), probable (23.2%), possible (23.2%), and doubtful (3.5%).
CONCLUSIONS. The analysis showed that most ADR reports were submitted in due time and in the scope stipulated by regulatory documents. In order to correctly assess the accuracy of ADR reports, the form should include as much data on the patient, medicinal products, and ADR as possible. Regularly improving staff awareness of pharmacovigilance procedure and reporting deadlines will increase pharmacovigilance effectiveness of a medical organisation.
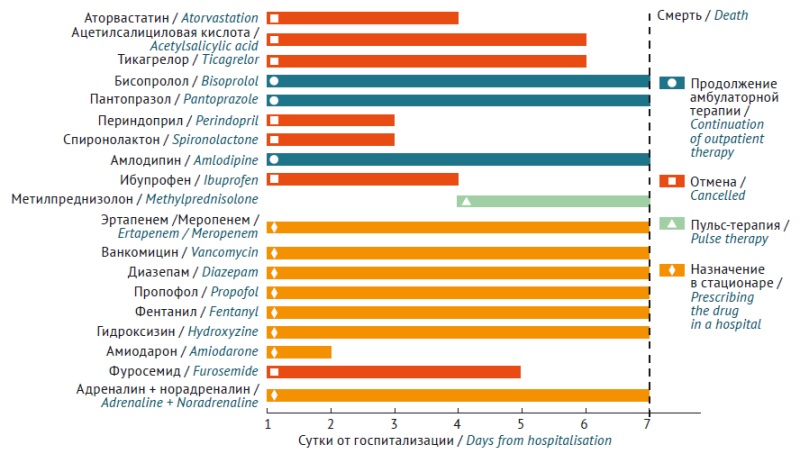
INTRODUCTION. Following acute coronary syndrome (ACS), patients are at high risk of repeated cardiovascular accidents. They receive intensive lipid-lowering and antiplatelet therapy according to clinical recommendations. However, therapy intensification may entail increased risks of adverse drug reactions. The clinical case describes fatal rhabdomyolysis associated with high-dose rosuvastatin therapy. The risk factors of this adverse reaction have been analysed; knowing the factors can help prevent similar events in patients.
CASE REPORT. A 68-year-old patient, male, received continuous therapy with rosuvastatin 10 mg per day for 3 years with good tolerability. After the ACS, rosuvastatin dose was increased to a maximum of 40 mg per day, dual antiplatelet therapy with ticagrelor was prescribed, as well as bisoprolol, amlodipine, omeprazole, perindopril, and spironolactone. Within a month, the patient developed muscle pain and acute renal failure, with clinical and laboratory evidence confirming rhabdomyolysis. Despite intensive therapy, the patient died. An analysis was performed for genetic markers of individual rosuvastatin pharmacokinetics, showing: CYP2C9 *1*1 (normal activity), SLCO1B1 *5*15 (reduced activity for homozygous state), ABCG2 c.421 C/C (normal activity). Literature analysis of drug interaction revealed possible additional increase in rosuvastatin concentrations (up to 2.6 times) associated with ticagrelor inhibiting breast cancer resistant protein transporter activity.
CONCLUSIONS. In the present case, fatal statin-associated rhabdomyolysis developed due to two significant factors — pharmacogenetic predisposition and a significant drug-drug interaction of rosuvastatin with ticagrelor, which disrupted the functions of two carrier proteins that determine medicine bioavailability (breast cancer resistant protein) and its transport through the hepatocyte membrane (OATPB1). Pharmacogenetic testing and active monitoring of laboratory values is indicated in such patients in the first days of drug therapy for the timely diagnosis of possible complications; such situations are crucial for the prognosis in patients after ACS following high-dose statin therapy and other medicines with the potential for significant drug interactions.
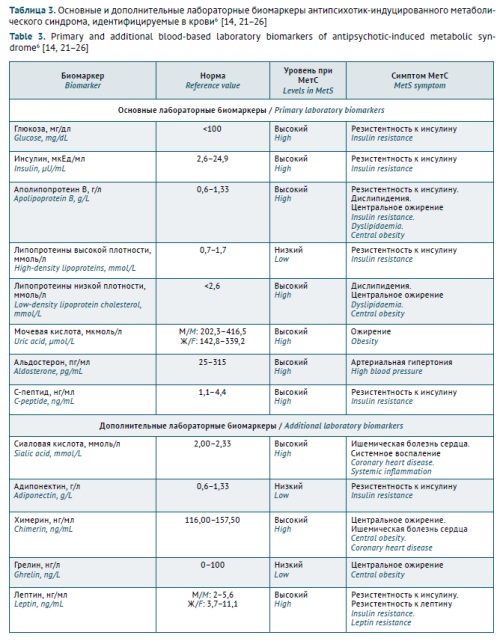
INTRODUCTION. Antipsychotic-induced metabolic syndrome (AIMetS) is a common adverse reaction to the pharmacotherapy of psychiatric and addiction disorders. However, interindividual variability in the metabolism of antipsychotics may limit the sensitivity and specificity of known blood-based biochemical biomarkers of AIMetS for assessing the safety of psychopharmacotherapy and the risk of AIMetS in patients with schizophrenia spectrum disorders. In recent years, circulating microRNAs have been considered as new and promising epigenetic biomarkers of AIMetS.
AIM. This study aimed to evaluate the potential of circulating microRNAs as epigenetic biomarkers for the prediction and early diagnosis of AIMetS.
DISCUSSION. The authors analysed the results of academic and clinical research published from 2012 to 2024 with a focus on the role of circulating microRNAs involved in the key AIMetS pathogenesis and progression pathways. This review presents novel international approaches to using primary and additional clinical and biochemical biomarkers of AIMetS and demonstrates the advantages of microRNAs as epigenetic biomarkers of AIMetS. The article summarises data on the roles of microRNAs in the mechanisms of AIMetS development (oxidative stress, systemic inflammation, adipocyte differentiation, lipid and glucose metabolism, appetite regulation, and changes in neuropeptide Y and orexin expression, leptin sensitivity, and testosterone, thyroid and parathyroid hormone levels).
CONCLUSIONS. Detecting changes in the expression of circulating microRNAs in easily accessible samples (blood, saliva, urine, etc.) is a promising alternative method for predicting and diagnosing AIMetS. The second part of this review will explore the role of circulating microRNAs as epigenetic biomarkers for developing the main manifestations of MetS and AIMetS and will classify microRNA signatures according to the risk of developing AIMetS.
ISSN 2619-1164 (Online)